Researchers in Texas are using rotaxane formation to sterically protect or “insulate” molecular wires.
Molecular wires, in which an unsaturated linker separates two or more redox active metal sites, are of great research interest. These structures allow phenomena such as electron delocalisation or transport between the two redox sites. John Gladysz’s group at Texas A&M University have an ongoing interest in dimetallic polyynediyl complexes, in which two metal centres are linked by conjugated polyynediyl linkers that they now hope to “insulate” to reduce interactions between wires and the external environment. A previous approach used long alkyl bis-phosphine, which wrapped around the wire in a double helix to complex both metal centres. However, this gave two enantiomers, which interconverted rapidly in solution via uncoiling of the protective ligands.
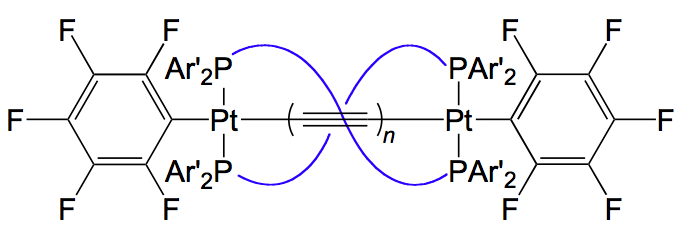
The Gladysz group are now reporting a straightforward solution to this problem. They found that by synthesising their bis-platinum wire in the presence of a 33-membered macrocycle, they could incorporate the wire as the thread of a rotaxane complex. This provides a more robust protection for the wire which is unaffected by dynamic processes.
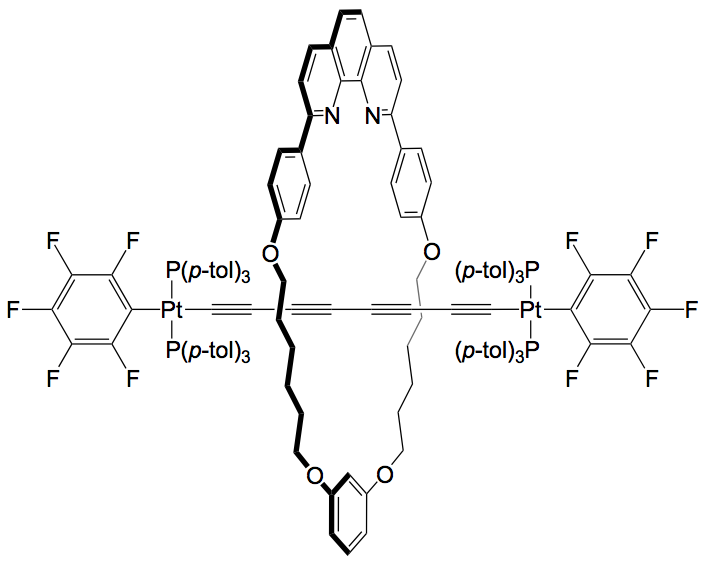 |
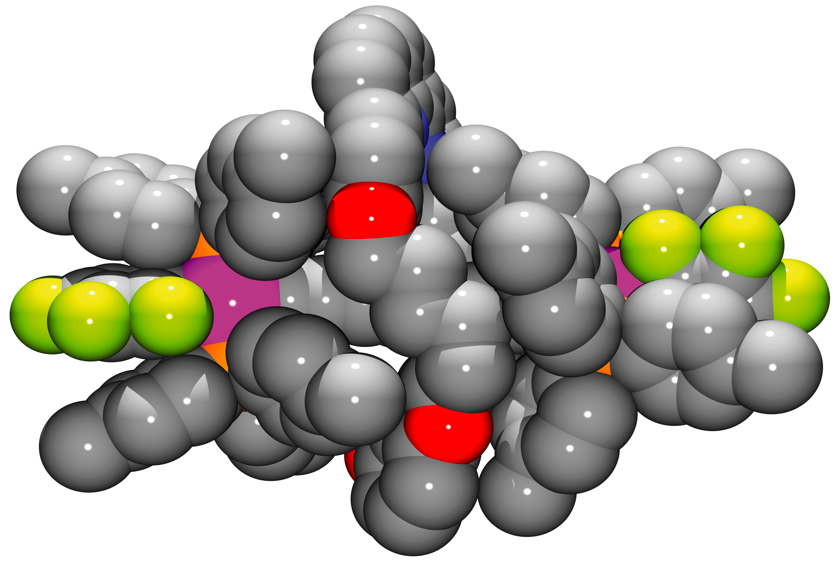 |
This work shows a fantastic application of rotaxane chemistry for protection of a molecular wire. What’s more, the synthesis of this rotaxane is adaptable, and the Gladysz group are working on exciting new and improved systems including longer polyynediyl linkers and redox inactive macrocycles to improve the properties of the insulated wires.
The full communication can be downloaded here.
Cally Haynes