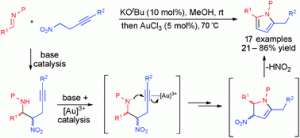
Catalytic reaction cascades present a neat, efficient way to synthesise valuable organic molecules. However, their success relies upon the compatibilty of the reactants and catalysts involved, and identifying this requires ingenuity and experimentation.
Now Darren Dixon and co-workers at the University of Oxford have reported a one-pot nitro-Mannich/hydroamination cascade for direct synthesis of 2,5-disubstituted pyrroles from imines and nitro alkynes. The reaction is catalysed by base and a gold(I) catalyst, and has been optimised to achieve yields up to 86%.
To find out more download the ChemComm communication, which is free to access until April 28th
Start a discussion by leaving your comments below