US, UK and Korean scientists have made a new porous phosphine coordination material, PCM-11. The material is an unusual 8,4-connected coordination polymer with an open 3-D pore structure, say the researchers.
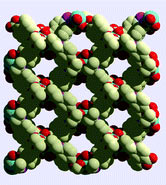 The team formed the material by reacting Mg(II) with tris(para-carboxylato)triphenylphosphine oxide. The highly ionic nature of the metal–ligand bonding results in excellent thermal stability upon desolvation (>460 ºC), they say. PCM-11 is easily activated for small molecule sorption at low temperature without the requirement for solvent pre-exchange. It adsorbs 47.5 wt% CO2 at 11.6 bar and 30ºC.
The team formed the material by reacting Mg(II) with tris(para-carboxylato)triphenylphosphine oxide. The highly ionic nature of the metal–ligand bonding results in excellent thermal stability upon desolvation (>460 ºC), they say. PCM-11 is easily activated for small molecule sorption at low temperature without the requirement for solvent pre-exchange. It adsorbs 47.5 wt% CO2 at 11.6 bar and 30ºC.
Find out more in the ChemComm communication:
High capacity CO2 adsorption in a Mg(II)-based phosphine oxide coordination material
Alisha M. Bohnsack, Ilich A. Ibarra, Peter W. Hatfield, Ji Woong Yoon, Young Kyu Hwang, Jong-San Chang and Simon M. Humphrey, Chem. Commun., 2011, DOI: 10.1039/C1CC10754B










