The development of antibacterial materials that can guard against microbial infections during medical procedures has been a topic of increasing interest. In this paper, a team of researchers from Beijing University of Chemical Technology and the University of Bremen describe a facile synthesis method for a hybrid nanomaterial that has previously demonstrated excellent antibacterial activity: reduced graphene oxide films decorated with silver nanoparticles. The efficient, dimensionally scalable, and environmentally friendly nature of this method make it a potentially valuable tool for other groups interested in fabricating these hybrid films for applications ranging from medical biomaterials, to cell culture scaffolds, to drug delivery platforms, to environmental remediation.
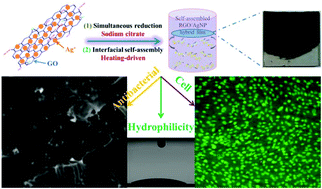
- Schematic model for fabricating RGO/AgNP hybrid film
Zhang et al. created these hybrid films by using sodium citrate to simultaneously reduce aqueous graphene oxide (GO) and aqueous silver nitrate to reduced graphene oxide (RGO) and silver nanoparticles (AgNPs), respectively. This was followed by a prolonged incubation at 80°C to induce thermal evaporation-driven self-assembly of the hybrid RGO/AgNP films. The mass ratio of silver nitrate to GO in the initial solution could be adjusted to produce films with varying densities of AgNPs. In addition, this mass ratio as well the thermal evaporation duration provided some control over the thickness of the resulting hybrid film. The film’s size could be readily changed via adjustments to the dimensions of the reaction container and the film could be later transferred to other substrates without any additional post processing steps.
Subsequent characterisation of the film confirmed the successful reduction of GO as well as the formation of primarily spherical AgNPs between 12 and 30 nm in size on the surface of the film. The resulting material was found to be very hydrophilic and supported the adhesion and proliferation of mouse osteoblasts. An antibacterial assay measuring E. coli adhesion on the surface of silicon wafers coated with these RGO/AgNP films found that the few bacteria that did manage to adhere to the film’s surface exhibited damaged cell walls. A subsequent E. coli colony viability assay proved that no living bacterial colonies were present on the surface of the hybrid film, a result that could not be obtained when using either an uncoated silicon wafer or a pure RGO film.
The biocompatibility, hydrophilicity, durability, and antibacterial activity of these RGO/AgNP hybrid films make them intriguing candidates for a number of medical and environmental applications. The simple, dimension-scalable, and environmental friendly synthesis method presented by Zhang et al. in this paper opens up new avenues for the creation and widespread use of these versatile hybrid films.
Check out the full article:
Graphene film doped with silver nanoparticles: self-assembly formation, structural characterizations, antibacterial ability, and biocompatibility by Panpan Zhang, Haixia Wang, Xiaoyuan Zhang, Wei Xu, Yang Li, Qing Li, Gang Wei, and Zhiqiang Su
Ellen Tworkoski is a Web Writer for Biomaterials Science and is currently a graduate student in the biomedical engineering department at Northwestern University (Evanston, IL, US).
Follow the latest journal news on Twitter @BioMaterSci or go to our Facebook page.