This HOT article from Xiaojing Chen and co-workers of Wenzhou University, Zhejiang Province, China, looks at the analysis of enantiomeric excess. The enantiomeric forms of many compounds exert different physiological and therapeutic effects, which are an important consideration in pharmaceutical and agrochemical research, leading to large and increasing demands for single-enantiomer products.
Many existing approaches can be time-consuming and destructive, but NMR has wide applicability due to its high speed, reliable detection for quantitative and qualitative analysis. The drawback, however, is the need for an equivalent or excess amount of the chiral reagent required to give rise to signal splitting, and these reagents are usually expensive or difficult to obtain.
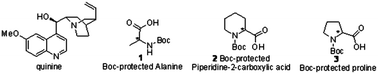
Here, the authors selected quinine, a commercially available and inexpensive reagent, as a chiral shift reagent to induce discrimination in the NMR spectra of enantio-isomers. They say their findings provide an accurate, rapid, and inexpensive method of determining the enantiomeric purity of amino acid derivatives, and show great potential in both peptide and pharmaceutical syntheses.
Communication: Rapid determination of enantiomeric excess of protected amino acids by catalytic amounts of chiral reagent
Xiangou Zhu, Jun Jiang, Xinxiang Lei and Xiaojing Chen
Anal. Methods, 2012, Advance Article
DOI: 10.1039/C2AY25102G
This paper will be free to access until 21 June 2012.