I was waiting for a train on a cold and rainy Friday morning in February at Preston train station (this will be a shock for those that know the Preston climate as it is actually subtropical) and tweeting from @ChemistryBaker to my colleagues @RoyGoodacre and @AlexHenderson00 about the upcoming kick off meeting of the Clinical Infrared and Raman Spectroscopy network. Analyst (@analystrsc) picked this tweet up and through a few tweets back and forth agreed to host a blog on the subject and here it is; to show a different side of research and the way a fledgling network starts and, hopefully, becomes successful. Success for us is closely linked to a clinical impact that would have a massive positive effect on many many people’s lives.
I was waiting for a train on a cold and rainy Friday morning in February at Preston train station (this will be a shock for those that know the Preston climate as it is actually subtropical) and tweeting from @ChemistryBaker to my colleagues @RoyGoodacre and @AlexHenderson00 about the upcoming kick off meeting of the Clinical Infrared and Raman Spectroscopy network. Analyst (@analystrsc) picked this tweet up and through a few tweets back and forth agreed to host a blog on the subject and here it is; to show a different side of research and the way a fledgling network starts and, hopefully, becomes successful. Success for us is closely linked to a clinical impact that would have a massive positive effect on many many people’s lives.
The UK is a world leader in the field of vibrational biomedical spectroscopy with excellent research being performed by many groups based in our academic and governmental research systems. Diagnostic and prognostic tools based on these technologies have the potential to revolutionise our clinical systems leading to improved patient outcome, a more efficient healthcare service and significant economic savings. However, despite these strong reasons and the research that has occurred to date, this technology has not truly made the jump into the clinic. There was a need to bring many researchers, clinicians and industrialists together in order to provide a critical mass that will overcome these clinical implementation barriers.
It was for these reasons, that in 2012, several of us got together to speak about the possibility of developing a bid for an EPSRC network grant. Importantly network grants provide no money for research projects rather funding to enable collaborative multidisciplinary meetings targeted to tackle problems and drive a field forwards. Luckily EPSRC agreed (thank you EPSRC) that our bid, plan and field deserved funding and the EPSRC Clinical Infrared and Raman SPECtroscopy (CLIRSPEC) network was set up.
Even though the meeting that February day was long and actually quite tiring it was also fun and welcoming. Our field is known to be a supportive field. Essentially the aims of the meeting was to get people together to discuss and finalise our plans to overcome the challenges we identified, agree how we assess bids to the training fund for PhDs and PDRAs, conference locations and summer school locations. The main aim was to ask already overworked people to do more work for free, which thankfully they agreed to.
The work of the network will be achieved through six working parties that aim to overcome the barriers that challenge our field:
1) To develop our understanding of the interaction of light with clinical samples
2) To develop internationally recognized protocols for the preparation of cells, tissue and biofluids for clinical spectroscopy
3) To provide evidence of the power of spectroscopy for use in the clinical arena
4) To determine the requirements of instrumentation suitable for use in the clinic
5) To develop strategies for pre-processing and statistical analysis in clinical spectroscopy
6) To develop inter-group data sharing protocols and portal
I would like to use this first blog to ask if there are any clinicians, scientists or industrialists out there who feel they can contribute to the cause. We currently have international and national partners and would welcome more. If you feel you can contribute then please visit our website. We do not want to be a closed shop but rather harness the power of many to achieve our aims.
Until the next time…
Please find links to recent papers below that describe the research currently on-going in the field to demonstrate the application of spectroscopy to the clinical environment as well as tackling the current research challenges.
The inherent problem of transflection-mode infrared spectroscopic microscopy and the ramifications for biomedical single point and imaging applications
Paul Bassan, Joe Lee, Ashwin Sachdeva, Juliana Pissardini, Konrad M. Dorling, John
S. Fletcher, Alex Henderson and Peter Gardner
Analyst, 2013,138, 144-157
A comparison of Raman, FTIR and ATR-FTIR micro spectroscopy for imaging human skin tissue sections
S. M. Ali, F. Bonnier, H. Lambkin, K. Flynn, V. McDonagh, C. Healy, T. C. Lee, F. M. Lyng and H. J. Byrne
Anal. Methods, 2013,5, 2281-2291
Effect of substrate choice and tissue type on tissue preparation for spectral histopathology by Raman microspectroscopy
Leanne M. Fullwood, Dave Griffiths, Katherine Ashton, Timothy Dawson, Robert W. Lea, Charles Davis, Franck Bonnier, Hugh J. Byrne and Matthew J. Baker
Analyst, 2014,139, 446-454
Identification of different subsets of lung cells using Raman microspectroscopy and whole cell nucleus isolation
Jacek K. Pijanka, Nicholas Stone, Abigail V. Rutter, Nicholas Forsyth, Ganesh D. Sockalingum, Ying Yang and Josep Sulé-Suso
Analyst, 2013,138, 5052-5058
ATR-FTIR spectroscopic imaging: recent advances and applications to biological systems
Sergei G. Kazarian and K. L. Andrew Chan
Analyst, 2013,138, 1940-1951
Illuminating disease and enlightening biomedicine: Raman spectroscopy as a diagnostic tool
David I. Ellis, David P. Cowcher, Lorna Ashton, Steve O’Hagan and Royston Goodacre
Analyst, 2013,138, 3871-3884
Simultaneous detection and quantification of three bacterial meningitis pathogens by SERS
Kirsten Gracie, Elon Correa, Samuel Mabbott, Jennifer A. Dougan, Duncan Graham, Royston Goodacre and Karen Faulds
Chem. Sci., 2014,5, 1030-1040
Resonance Raman microscopy in combination with partial dark-field microscopy lights up a new path in malaria diagnostics
Bayden R. Wood, Antje Hermelink, Peter Lasch, Keith R. Bambery, Grant T. Webster, Mehdi Asghari Khiavi, Brian M. Cooke, Samantha Deed, Dieter Naumann and Don McNaughton
Analyst, 2009,134, 1119-1125
Extracting biological information with computational analysis of Fourier-transform infrared (FTIR) biospectroscopy datasets: current practices to future perspectives
Júlio Trevisan, Plamen P. Angelov, Paul L. Carmichael, Andrew D. Scott and Francis L. Martin
Analyst, 2012,137, 3202-3215
Emerging concepts in deep Raman spectroscopy of biological tissue
Pavel Matousek and Nicholas Stone
Analyst, 2009,134, 1058-1066
Vibrational spectroscopy: a clinical tool for cancer diagnostics
Catherine Kendall, Martin Isabelle, Florian Bazant-Hegemark, Joanne Hutchings, Linda Orr, Jaspreet Babrah, Rebecca Baker and Nicholas Stone
Analyst, 2009,134, 1029-1045
Also please see the latest themed issue on Optical Diagnostics











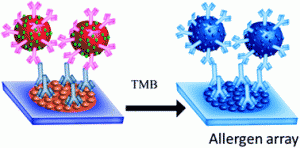 nt surveys from Allergy UK have shown that the rates of allergy are increasing throughout the world, affecting up to 30-35 % of people at some point during their lives, in some cases with fatal consequences. In vitro testing of specific Immunoglobin E (IgE) reactivities is thought to be able to significantly improve diagnostic accuracy and management in primary care. IgE plays a significant role in allergic conditions. Current methods for the sensitive and accurate detection of specific IgE reactivities are expensive; require significant sample preparation and investment in equipment. There is a need to provide a cheap, rapid, sensitive and specific test for IgE reactivities.
nt surveys from Allergy UK have shown that the rates of allergy are increasing throughout the world, affecting up to 30-35 % of people at some point during their lives, in some cases with fatal consequences. In vitro testing of specific Immunoglobin E (IgE) reactivities is thought to be able to significantly improve diagnostic accuracy and management in primary care. IgE plays a significant role in allergic conditions. Current methods for the sensitive and accurate detection of specific IgE reactivities are expensive; require significant sample preparation and investment in equipment. There is a need to provide a cheap, rapid, sensitive and specific test for IgE reactivities.